Corneal cross-linking (CXL), in addition to saturating the structural layer of the cornea – the stroma – with riboflavin, also involves irradiating this region with ultraviolet (UV) light. This causes a photochemical reaction to occur in the stroma, which cross-links together these stromal molecules, resulting in the stroma becoming stiffer and stronger. Although the stroma is essentially just collagen and proteoglycans (with almost no living cells located there), there are also cell-dense layers above and below, and these can be damaged by UV light.
Why Standard CXL Poses Risks in Thin Corneas
The layer of epithelial cells above the stroma acts as a barrier to protect the cornea from the environment. Epithelial cells are commonly used during traditional CXL protocols in order to let riboflavin penetrate the stroma. However, these cells quickly regrow and repopulate the cornea in the days after the procedure.
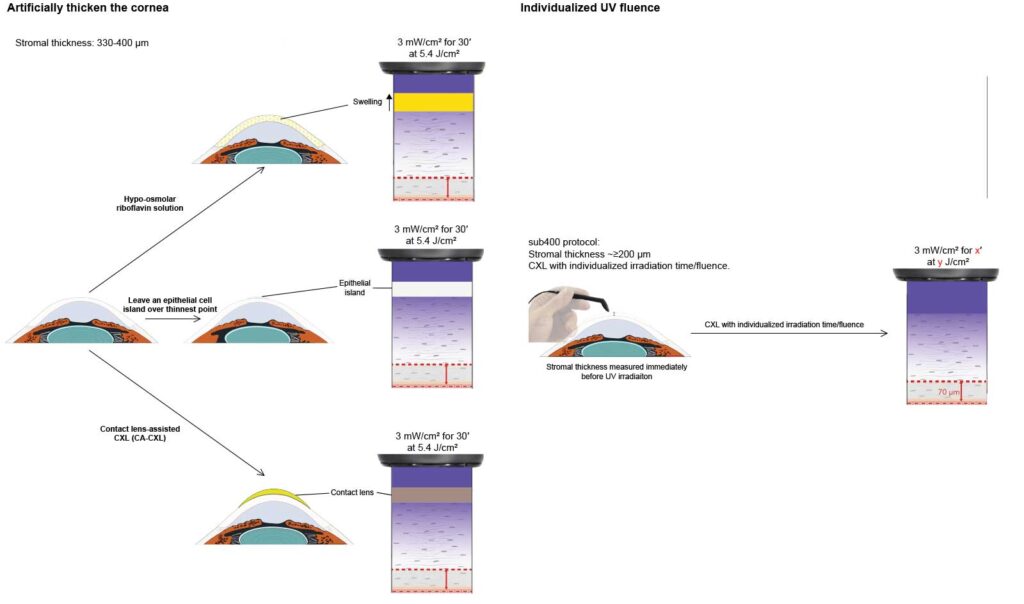
Several modifications to the Dresden protocol followed: swelling the cornea with hypoosmolar riboflavin to artificially thicken it; adding a riboflavin-soaked contact lens, and also leaving some epithelial cells intact over the thinnest parts of the cornea. All had drawbacks. Respectively, these were: unpredictable swelling effects; low stiffening efficacy, and problems with UV light scatter at the interface between regions with and without epithelial cells.
ELZA-sub400: A Fluence-Adapted Approach
ELZA developed the ELZA-sub400 protocol, which took a different approach. Instead of modifying the cornea to fit the technique, which these previous approaches all attempted, we adapted the technique to the cornea with a beautifully simple concept: simply cross-link the stroma down to the 70 µm safety limit. In reality, this involved years of work to build a mathematical model of how UV light, riboflavin, stromal tissue, and oxygen interact, and then preclinical work to validate it before the first patients could receive ELZA-sub400 CXL. And to be fair, ELZA-sub400 CXL works. However, there was one question to be answered: does it have any effect on the corneal endothelium?
Assessing the Effect on the Endothelium
A newly published clinical study in the Journal of Refractive Surgery now confirms the long-term sub400 endothelial safety of the ELZA-sub400 protocol, a fluence-adapted, pachymetry-guided CXL approach developed specifically for ultra-thin corneas.
This investigator-initiated, single-centre study was conducted at the University Hospital Zurich in collaboration with the ELZA Institute. Seventeen eyes with documented progression and minimum stromal thicknesses as low as 210 µm underwent CXL using the ELZA-sub400 protocol. Fluence was adjusted based on intraoperative pachymetry, ensuring a minimum buffer zone between the cross-linked stroma and the endothelium.
Endothelial Safety Confirmed Over 24 Months
The ELZA-sub400 protocol is based on physical principles—namely Lambert-Beer’s law of UV attenuation and Fickian diffusion of riboflavin and oxygen. Rather than attempting to artificially swell the cornea or add procedural complexity, ELZA-sub400 adjusts UV-A exposure time according to the patient’s true stromal thickness, preserving biomechanical efficacy while safeguarding endothelial cells.
A Standardised Solution for High-Risk Corneas?
These clinical data follow earlier ex vivo and modelling studies and mark the first long-term confirmation of ELZA-sub400’s endothelial safety in a real-world population. Notably, no intra- or postoperative complications were reported, and the structural integrity of the corneal endothelium remained intact across all follow-up intervals.
What This Means for Clinical Practice
Reference
Blaser F, Torres-Netto EA, Gatzioufas Z, Perschak P, Hafezi F, Said S. Assessing endothelial integrity in patients with progressive keratoconus and thin corneas treated with the sub400 corneal cross-linking protocol. J Refract Surg. 2025;41(7):e682-e689. PMID: 40626429