Over the past decade, the role of corneal cross-linking (CXL) in keratoconus management has evolved substantially. What began as a method to halt disease progression has developed into a family of increasingly refined, individualized treatment strategies. In a 2024 review, Prof. Farhad Hafezi, MD, PhD, FARVO summarizes the most relevant advances shaping modern CXL practice in an article in ZPA.
Corneal cross-linking advances
The epithelium-off “Dresden protocol” remains the historical foundation of CXL. By removing the epithelium, saturating the stroma with riboflavin, and applying UV-A irradiation at 3 mW/cm² for 30 minutes, this protocol reliably increases corneal biomechanical stability. However, its duration and postoperative morbidity have motivated efforts to optimize treatment efficiency without compromising efficacy.
Accelerated CXL protocols emerged with the introduction of modern LED UV-A light sources, aiming to deliver equivalent fluence over shorter treatment times. Early attempts revealed an important limitation: oxygen depletion can reduce biomechanical stiffening when fluence is delivered too rapidly. Building on this insight, research from Zurich demonstrated that higher total fluence protocols—such as 10 J/cm² delivered over just over nine minutes—can achieve biomechanical effects comparable to the Dresden protocol, while substantially reducing treatment time. These approaches may be particularly relevant in pediatric or advanced cases where a stronger effect is desired.
Managing thin and ultrathin corneas remains a central challenge in keratoconus care. Traditional epithelium-off CXL is contraindicated below a stromal thickness of 400 µm due to endothelial safety concerns. The individualized ELZA-sub400 protocol addresses this limitation by adjusting UV fluence according to measured stromal thickness, enabling safe CXL in corneas as thin as 180 µm. Clinical validation has provided a standardized framework for treating eyes that were previously considered unsuitable for cross-linking.
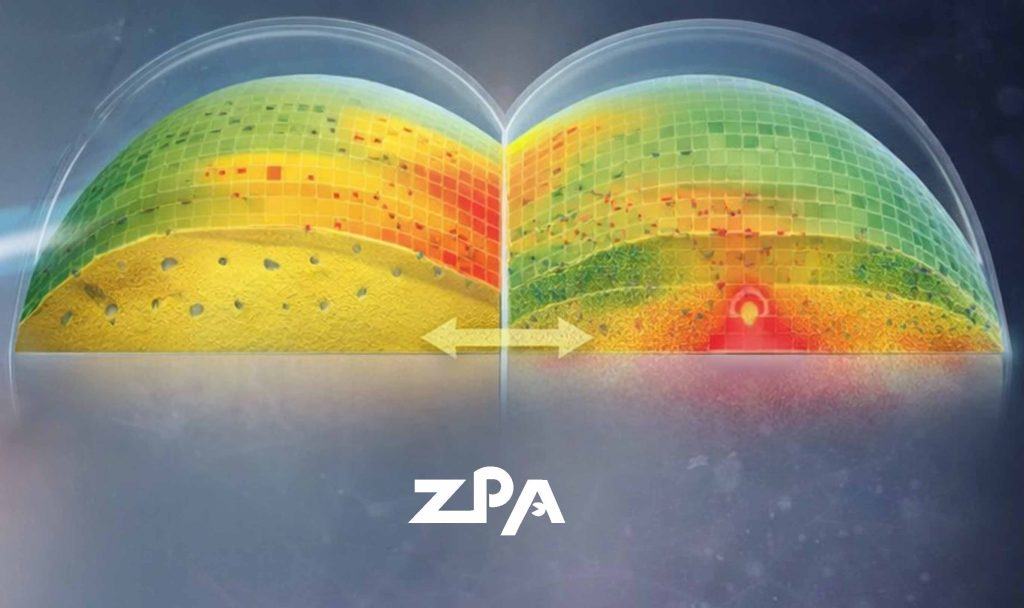
Parallel efforts have focused on transepithelial (epi-on) CXL to reduce pain and infection risk associated with epithelial removal. Advances in riboflavin formulations and irradiation strategies have made it possible to achieve clinically meaningful stiffening without additional oxygen supplementation or iontophoresis.
A further step toward personalization is second-generation customized CXL. ELZA-PACE combines epithelial mapping–guided phototherapeutic keratectomy over the cone apex with customized epi-on CXL, creating controlled gradients of riboflavin concentration, oxygen availability, and UV fluence. This approach has demonstrated greater cone flattening than earlier customized protocols, without the need for complex eye-tracking systems.
Together, these developments reflect a broader shift in keratoconus management: from uniform stabilization toward patient-specific biomechanical and refractive optimization, guided by corneal thickness, topography, and disease stage.